Fungicides-late blight interaction in the synthesis of phenolic compounds and defense enzyme activity in tomato
DOI:
https://doi.org/10.31285/AGRO.28.1434Keywords:
Phytophthora infestans, Solanum lycopersicum, fluoxastrobin, fosetyl-AlAbstract
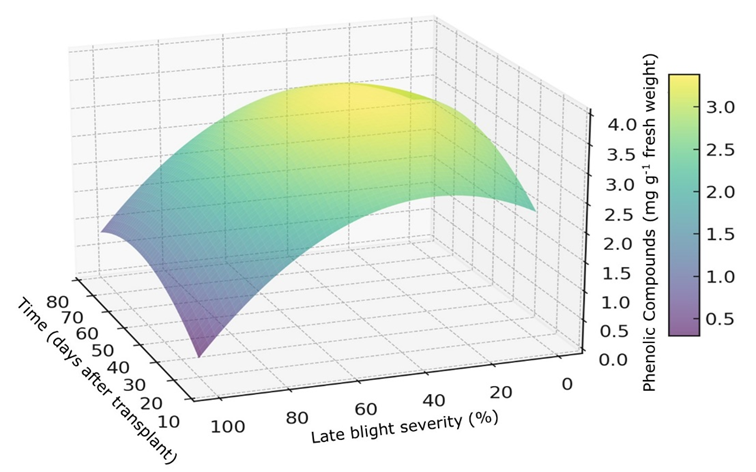
Due to the significant impact of late blight (LB) (Phytophthora infestans [Mont.]) on tomato (Solanum lycopersicum L.), we investigated the interaction between fungicides and this disease to understand how some plant defense mechanisms are affected over time. Following a randomized design, we evaluated the synthesis of phenolic compounds (PHE) and the activity of phenylalanine ammonium lyase (PAL), peroxidases (POX) and superoxide dismutase (SOD). The experiment involved the application of fosetyl-Al and fluoxastrobin (fungicides with dual modes of action) on healthy and infected tomato plants. LB severity was assessed weekly and leaf samples were collected at various intervals for biochemical analysis. The Kruskal-Wallis test (α = 0.05) analyzed main effects of infection, fungicide, and time on response variables, followed by Bonferroni post hoc for significant group differences and regression models to evaluate variable effects over time. The application of fungicides had no effect on enzymatic activity or PHE accumulation. While PAL and SOD activities were not significantly affected by infection, POX activity was significantly higher in healthy plants (4793.8 U g-1 fresh weight) compared to infected plants (1858.1 U g-1 fresh weight). A complex interaction between PHE accumulation in relation to LB severity and time was observed, with a notable increase in PHE levels at 50 days after transplant when disease severity was between 25 and 50%. Future studies should consider including a broader range of genotypes and isolates of P. infestans, a more extensive set of biochemical responses, and evaluations of the overexpression of genes related to plant defense.
Downloads
References
Alia-Tejacal I, Colinas-León MT, Martínez-Damián MT, Soto-Hernández MR. Actores fisiológicos, bioquímicos yde calidad en frutos de Zapote Mamey (Pouteria sapota Jacq. H.E. Moore & Stearn) durante poscosecha. Rev Chapingo Ser Hortic. 2002;8(2):263-81. Doi: 10.5154/r.rchsh.2001.11.083. DOI: https://doi.org/10.5154/r.rchsh.2001.11.083
Almagro L, Gómez Ros LV, Belchi-Navarro S, Bru R, Ros Barceló A, Pedreño MA. Class III peroxidases in plant defence reactions. J Exp Bot. 2009;60(2):377-90. Doi: 10.1093/jxb/ern277. DOI: https://doi.org/10.1093/jxb/ern277
Anand T, Chandrasekaran A, Kuttalam S, Raguchander T, Prakasam V, Samiyappan R. Association of some plant defense enzyme activities with systemic resistance to early leaf blight and leaf spot induced in tomato plants by azoxystrobin and Pseudomonas fluorescens. J Plant Interact. 2007;2(4):233-44. Doi: 10.1080/17429140701708985. DOI: https://doi.org/10.1080/17429140701708985
Attia MS, Hashem AH, Badawy AA, Abdelaziz AM. Biocontrol of early blight disease of eggplant using endophytic Aspergillus terreus: Improving plant immunological, physiological and antifungal activities. Bot Stud. 2022;63(1):26. Doi: 10.1186/s40529-022-00357-6. DOI: https://doi.org/10.1186/s40529-022-00357-6
Becktell MC, Daughtrey ML, Fry WE. Epidemiology and Management of Petunia and Tomato Late Blight in the Greenhouse. Plant Dis. 2005;89(9):1000-8. Doi: 10.1094/PD-89-1000. DOI: https://doi.org/10.1094/PD-89-1000
Beyer WF Jr, Fridovich I. Assaying for superoxide dismutase activity: some large consequences of minor changes in conditions. Anal Biochem. 1987;161(2):559-66. Doi: 10.1016/0003-2697(87)90489-1. DOI: https://doi.org/10.1016/0003-2697(87)90489-1
Bos JI, Kanneganti TD, Young C, Cakir C, Huitema E, Win J, Armstrong MR, Birch PR, Kamoun S. The C-terminal half of Phytophthora infestans RXLR effector AVR3a is sufficient to trigger R3a-mediated hypersensitivity and suppress INF1-induced cell death in Nicotiana benthamiana. Plant J. 2006;48(2):165-76. Doi: 10.1111/j.1365-313X.2006.02866.x. DOI: https://doi.org/10.1111/j.1365-313X.2006.02866.x
de Jong M, van den Ackerveken G. Fungal and Oomycete Biotrophy. In: Molecular Aspects of Plant Disease Resistance. Oxford: Wiley-Blackwell; 2018. Doi: 10.1002/9781119312994.apr0364. DOI: https://doi.org/10.1002/9781119312994.apr0364
Debona D, Rodrigues FA. A Strobilurin Fungicide Relieves Bipolaris oryzae-Induced Oxidative Stress in Rice. J Phytopathol. 2016;164(9):571-81. Doi: 10.1111/jph.12481. DOI: https://doi.org/10.1111/jph.12481
Di Marco S, Osti F, Calzarano F, Roberti R, Veronesi A, Amalfitano C. Effects of grapevine applications of fosetyl-aluminium formulations for downy mildew control on "esca" and associated fungi. Phytopathol Mediterr. 2011;50:S285-S299. DOI: https://doi.org/10.36253/phyto-5457
Flurkley WH, Jen JJ. Peroxidase and polyphenol oxidase activities in developing peaches. J Food Sci. 1978;43(6):1826-8. Doi: 10.1111/j.1365-2621.1978.tb07424.x. DOI: https://doi.org/10.1111/j.1365-2621.1978.tb07424.x
Forbes GA, Morales JG, Restrepo S, Pérez W, Gamboa S, Ruiz R, Cedeno L, Fermin G, Andreu AB, Acuna I, Oliva R. Phytophthora infestans and Phytophthora andina on Solanaceous hosts in South America. In: Lamour K, editors. Phytophthora: A global perspective. Oxfordshire: CABI; 2013. p. 48-58. DOI: https://doi.org/10.1079/9781780640938.0048
Fritz V, Tereucán G, Santander C, Contreras B, Cornejo P, Ferreira PAA, Ruiz A. Effect of Inoculation with Arbuscular Mycorrhizal Fungi and Fungicide Application on the Secondary Metabolism of Solanum tuberosum Leaves. Plants (Basel). 2022;11(3):278. Doi: 10.3390/plants11030278. DOI: https://doi.org/10.3390/plants11030278
Gill SS, Tuteja N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol Biochem. 2010;48(12):909-30. Doi: 10.1016/j.plaphy.2010.08.016. DOI: https://doi.org/10.1016/j.plaphy.2010.08.016
Henfling JW. El tizón tardfo de la papa: Phytophthora infestans. Lima: Centro Internacional de la Papa; 1987. 25p.
Ivanov AA, Ukladov EO, Golubeva TS. Phytophthora infestans: An Overview of Methods and Attempts to Combat Late Blight. J Fungi (Basel). 2021;7(12):1071. Doi: 10.3390/jof7121071. DOI: https://doi.org/10.3390/jof7121071
Kuzniak E, Skłodowska M. Fungal pathogen-induced changes in the antioxidant systems of leaf peroxisomes from infected tomato plants. Planta. 2005;222(1):192-200. Doi: 10.1007/s00425-005-1514-8. DOI: https://doi.org/10.1007/s00425-005-1514-8
Leadbeater A, Staub T. Exploitation of Induced Resistance: A Commercial Perspective. In: Walters DR, Newton AC, Lyon GD, editors. Induced Resistance for Plant Defense. Oxford: Wiley Blackwell; 2014. p. 300-15. Doi: 10.1002/9781118371848.ch13. DOI: https://doi.org/10.1002/9781118371848.ch13
Lozoya-Saldaña H, Rivera-Hinojosa R, Colinas-León MT. Fenoles, Peroxidasa y fenilalanina amonio-lyasa: Su relación con la resistencia genética de clones de papa (Solamun tuberosum L.) contra el tizón tardío (Phytophthora infestans Mont. De Bary). Agrociencia [Internet]. 2007 [cited 2024 May 29];41(4):479-89. Availabe from: https://www.scielo.org.mx/scielo.php?script=sci_arttext&pid=S1405-31952007000400479
Lyon GD. Agents That Can Elicit Induced Resistance. In: Walters DR, Newton AC, Lyon GD, editors. Induced resistance for plant defense. Oxford: Wiley Blackwell; 2014. p. 11-40. Doi: 10.1002/9781118371848.ch2. DOI: https://doi.org/10.1002/9781118371848.ch2
Martínez-Téllez MA, Lafuente MT. Effect of high temperature conditioning on ethylene, phenylalanine ammonia-lyase, peroxidase and polyphenol oxidase activities in flavedo of chilled ‹Fortune› mandarin fruit. J Plant Physiol. 1997;150(6):674-8. Doi: 10.1016/S0176-1617(97)80282-9. DOI: https://doi.org/10.1016/S0176-1617(97)80282-9
Nascimento J, Barrigossi JA. O papel das enzimas antioxidantes na defesa das plantas contra insetos herbívoros e fitopatógenos. Agrarian Academy [Internet]. 2014 [cited 2024 May 29];1(01):234-50. Availabe from: https://conhecer.org.br/ojs/index.php/agrarian/article/view/5225 DOI: https://doi.org/10.18677/Agrarian_Academy_2014_021
Ninkuu V, Yan J, Fu Z, Yang T, Ziemah J, Ullrich MS, Kuhnert N, Zeng H. Lignin and Its Pathway-Associated Phytoalexins Modulate Plant Defense against Fungi. J Fungi (Basel). 2022;9(1):52. Doi: 10.3390/jof9010052. DOI: https://doi.org/10.3390/jof9010052
Nowicki M, Foolad MR, Nowakowska M, Kozik EU. Potato and Tomato Late Blight Caused by Phytophthora infestans: An Overview of Pathology and Resistance Breeding. Plant Dis. 2012;96(1):4-17. Doi: 10.1094/PDIS-05-11-0458. DOI: https://doi.org/10.1094/PDIS-05-11-0458
Pirondi A, Brunelli A, Muzzi E, Collina M. Post-infection activity of fungicides against Phytophthora infestans on tomato (Solanum lycopersicum L.). J Gen Plant Pathol. 2017;83:244-52. Doi: 10.1007/s10327-017-0717-8. DOI: https://doi.org/10.1007/s10327-017-0717-8
Robledo-Esqueda MN, Saldaña HL, León MTC. Inducción de defensa en papa (Solanum tuberosum L.) CONTRA Phytophthora infestans Mont. de Bary por fungicidas. Interciencia [Internet]. 2012 [cited 2024 May 29];37(9):689-95. Availabe from: https://www.interciencia.net/wp-content/uploads/2018/01/689-c-LOZOYA-7.pdf
Saeidian S, Ghasemifar E. Effect of Temperature on Guaiacol Peroxidase of Pyrus communis. Int Lett Nat Sc. 2013;5:46-51. Doi: 10.56431/p-k4l209. DOI: https://doi.org/10.18052/www.scipress.com/ILNS.5.46
Serrano-Cervantes R, Lozoya-Saldaña H, Colinas y León MTB, Leyva-Mir SG. Algunas alteraciones enzimáticas en papa causadas por fungicidas. Rev Fitotec Mex. 2016;39(1):25-31. Availabe from: https://www.scielo.org.mx/pdf/rfm/v39n1/v39n1a6.pdf DOI: https://doi.org/10.35196/rfm.2016.1.25-31
Shakya SK, Larsen MM, Cuenca-Condoy MM, Lozoya-Saldaña H, Grünwald NJ. Variation in Genetic Diversity of Phytophthora infestans Populations in Mexico from the Center of Origin Outwards. Plant Dis. 2018;102(8):1534-40. Doi: 10.1094/PDIS-11-17-1801-RE. DOI: https://doi.org/10.1094/PDIS-11-17-1801-RE
Vallad GE. Tomato fungicides and other disease management products. In: Ozores-Hampton M, Snodgrass C, editors. Florida Tomato Institute Proceedings. Florida: University of Florida; 2011. p. 47.
Venancio WS, Rodrigues MAT, Begliomini E, de Souza NL. Physiological effects of strobilurin fungicides on plants. Publicatio UEPG. 2003;9(03). Doi: 10.5212/publicatio.v9i03.814.
Volke HV. Estimación de funciones de respuesta para información de tipo no experimental, mediante regresión. Montecillo: Colegio de Postgraduados; 2008. 113p.
Wang Y, Xu Y, Liu Z. A review of plant antipathogenic constituents: Source, activity and mechanism. Pestic Biochem Physiol. 2022;188:105225. Doi: 10.1016/j.pestbp.2022.105225. DOI: https://doi.org/10.1016/j.pestbp.2022.105225
Waterman PG, Mole S. Analysis on phenolic plant metabolites. Oxford: Blackwell Scientific Publications; 1994. 238p.
Xuanli J, Zhenqi L, Zhensheng K. The recent progress of research on peroxidase in plant disease resistance. J Northwest Sci-Tech Univ Agric For. 2001;29(6):124-9.
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2024 Agrociencia Uruguay

This work is licensed under a Creative Commons Attribution 4.0 International License.
| Article metrics | |
|---|---|
| Abstract views | |
| Galley vies | |
| PDF Views | |
| HTML views | |
| Other views | |